- Visibility 50 Views
- Downloads 14 Downloads
- DOI 10.18231/j.ijpns.2024.007
-
CrossMark
- Citation
Expectant management in symptomatic spontaneous pneumothorax in a newborn: A case report
Introduction
Pneumothorax occurs when air is accumulated between the visceral and parietal pleura. It may occur spontaneously or secondarily from underlying lung disease or the use of ventilator support. The underlying mechanism of spontaneous pneumothorax, particularly in term newborns is not fully understood. Newborns develop high negative transpulmonary pressure during the first few breaths to inflate the liquid-filled airway.[1], [2] Prolonged high transpulmonary pressure may predispose the alveolar to rupture, leading to pneumothorax.[3]
Symptomatic spontaneous pneumothorax occurs in 0.05-1% term newborns.[4], [5] Risk factors include male newborns, meconium aspiration syndrome (MAS), transient tachypnea of newborn (TTN) and respiratory distress syndrome (RDS).[4], [6], [7] Early recognition is crucial to prevent hypoxemia, hypercarbia and cardiovascular compromise. However, data regarding spontaneous neonatal pneumothorax is limited in Indonesia. In this study, we reported a case of spontaneous pneumothorax in a term newborn without history of resuscitation nor underlying lung pathology.
The case
A 40 weeks’ gestational age male baby was born to a 28-year-old primipara mother through caesarian section due to failed induction of labor. The baby cried immediately after birth, weighting 3150 grams with Apgar score of 9 and 10 at 1 and 5 minutes respectively. The initial assessment was done by pediatrician and no resuscitation was required. During pregnancy, the mother had regular antenatal check-ups with no perinatal risk factors. Within 1 hour after birth, the baby developed sudden respiratory distress with grunting, nasal flaring and subcostal retractions. The heart rate was 170 beats / minute, respiratory rate of 60 breaths / minute, saturation of 44% on room air with slight asymmetry chest movement during respiration. Oxygen saturation increased to 86%-90% with administration of oxygen via nasal prongs. The baby was immediately shifted from nursery room to neonatal intensive care unit (NICU) and continuous positive airway pressure (CPAP) with peep of 7 cmH2O and FiO2 of 0.21 was administered.
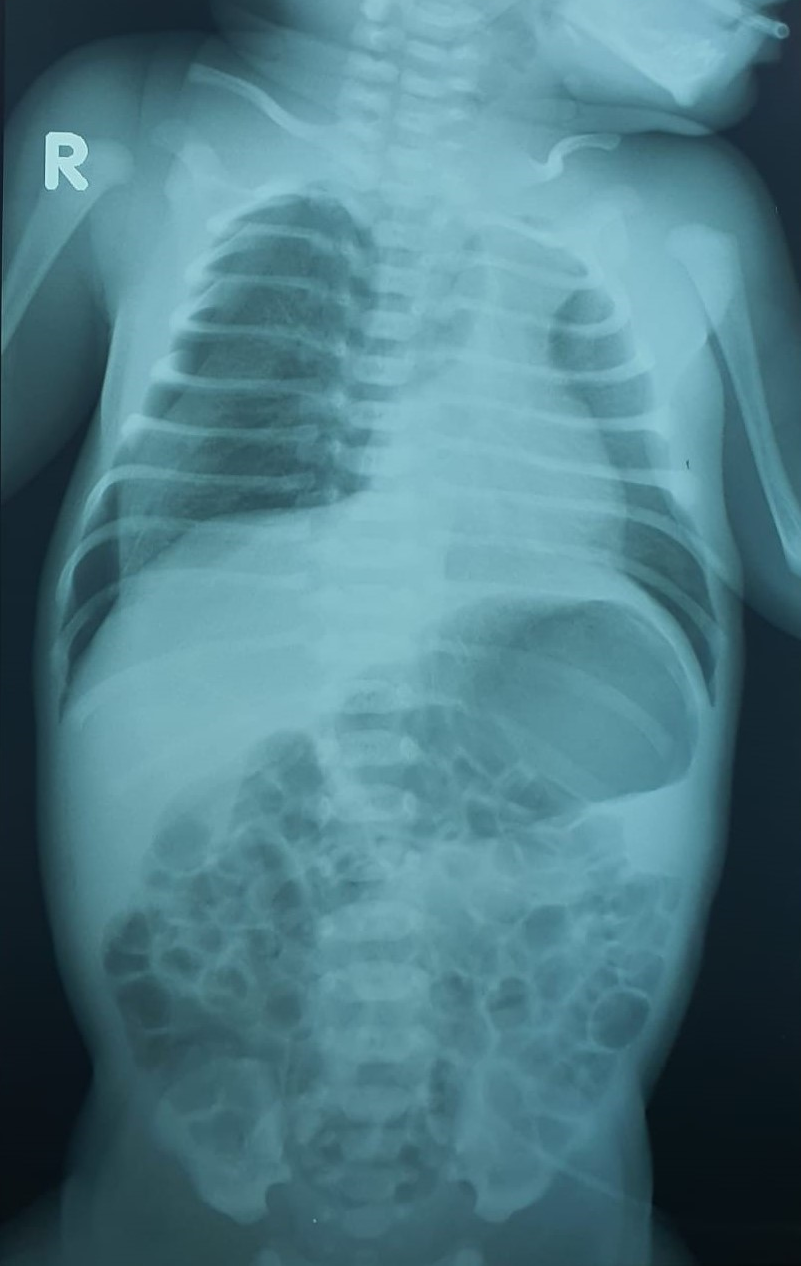
Physical evaluation showed lethargic newborn with subcostal retractions, asymmetry chest with right side bulging during each respiration and decreased right sided breathing on auscultation. The respiratory rate was 60 breaths / minute with saturation of 92%, heart rate of 140 beats / minute, capillary refill time 2 seconds with palpable peripheral pulses. The other physical examinations were within normal limit and the trachea was palpated on midline. The chest x-ray showed pneumothorax on the right side ([Figure 1]) while the initial laboratory work-up showed hemoglobin 14.2 g/dL, hematocrit 43 %, total leukocyte counts 41760/uL, thrombocyte count 285000/uL and blood glucose of 178 mg/dL. The baby was placed under careful observation and intravenous antibiotic was administered. Clinical improvements were observed within 24 hours and saturation was maintained between 94-98% and oxygen was put off after 48 hours. No signs of circulatory compromise occurred during observation. Repeated chest x-ray and septic screening revealed normal results on day-3 and day-6 respectively. The baby was discharged after 8 days of hospitalization.
Discussion
Spontaneous pneumothorax is one of rare air leak syndrome. Despite being relatively common in neonatal age group compared to overall pediatric population, studies reporting spontaneous pneumothorax in newborn remain scarce. Previous studies reported 0.5%-2% newborn had spontaneous pneumothorax and mostly asymptomatic, however the incidence in Indonesia is unknown.[4], [7], [8], [9], [10] Ramadanti et al. reported the prevalence of air leak syndrome including pneumothorax and pneumomediastinum was 0.02% in NICU of tertiary hospital in South Sumatra, Indonesia.[11] In this case report, we aim to describe a symptomatic spontaneous pneumothorax in term newborn in a hospital in Jakarta, Indonesia.
The development of neonatal pneumothorax could occur spontaneously or in association with precipitating factors. The collapsed fetal lung complicated by underlying disease might require higher negative transpulmonary pressure. Previous studies showed an increased incidence of secondary spontaneous pneumothorax in infant with RDS, MAS, TTN, and infant requiring resuscitation or respiratory support. Interventional deliveries including vacuum delivery and caesarian section were reported as risk factors.[4], [6], [7] Incomplete clearance of fetal lung fluid and relatively lower catecholamine level during caesarian were presumed to contribute in the pathogenesis.[1], [12], [13] Other reported risk factors were infant with male gender, larger or smaller birth weight, and preterm. [6], [7] Al Tawil et al. showed a group of infants with symptomatic spontaneous pneumothorax had significantly larger birth weight than control group (3,150 g ± 450 vs 2,750 ± 1,310) although both were in normal range. [4] The possible hypothesis was larger birth weight more likely to be born with interventional deliveries, due to high rate of vacuum deliveries in the study group. No prenatal risk factors were noted in our case report, consistent with other similar studies.[8], [14], [15]
The exact mechanism of spontaneous pneumothorax in term newborn is not fully understood. During the initial inspiratory effort of newborn to expand the lung, the generated negative pressure can be as high as 90cmH20. In comparison, two separated postmortem studies demonstrated lung rupture of human newborn and neonatal rabbit after subjected to a pressure of 70mmH20 and 45mmH20 respectively. At birth, some part of lung was either collapsed or partially inflated.[16] High inflating pressure during the initial breaths, may lead to an uneven distribution, causing rupture of overdistended alveoli.[1], [3]
Pneumothorax is a potentially life-threatening condition. Studies reported 7% of spontaneous pneumothorax progressed to tension pneumothorax. [10] Smith et al. reported the development of persistent pulmonary hypertension among 15% term and late preterm with spontaneous pneumothorax. [17] The earliest onset of spontaneous pneumothorax occurred immediately after birth, therefore early recognition with careful monitoring is crucial. Depending on the size and the risk factors, spontaneous pneumothorax might present with no symptoms to severe respiratory distress immediately after birth. Physical examination findings include sudden onset of respiratory distress, chest asymmetry, decreased breath sound on the affected side, and shifted apex position.[3], [4], [17] A clinician should highly consider pneumothorax when there is a sudden unexplained deterioration in oxygenation or cardiovascular status in mechanically ventilated infants. Supine chest x-ray provides definitive diagnosis of pneumothorax with increased lucency on the affected hemithorax. In case of uncertain radiographic signs, a lateral decubitus x-ray with the affected side up could further confirm the diagnosis. In large pneumothorax, a flattening of diaphragm and shift of the mediastinum can be visualized from chest x-ray.[3], [17], [18] Tension pneumothorax is suspected when there is collapse of the affected lung and mediastinal shift to the opposite side.[15], [17], [18]
The management of spontaneous pneumothorax include watchful waiting, needle thoracentesis or chest tube drainage. To date, no global consensus for the management of spontaneous pneumothorax in pediatric, particularly in neonatal population. Most asymptomatic, clinically stable patient with mild symptoms, or primary spontaneous pneumothorax were treated with careful observation and supportive management. While large pneumothorax and tension pneumothorax indicated chest drainage. Needle thoracentesis may be performed in clinically unstable patient as a rescue treatment before chest tube drainage.[3], [19] Reported complications of chest tube drainage were viscus perforation, bleeding, and catheter dislodgement.[9], [20] Clinical improvement of pneumothorax did not differ between expectant and active management with median time of 24-48 hours.[5], [6], [14], [18], [19]
Inhalation of 100% oxygen for nitrogen washout to increase the air reabsorption hastened the duration from 48 hours to 8-12 hours.[4] However, retinopathy of prematurity in term infants and risk of epithelium damage and impairment of mucociliary clearance were reported from exposure of high oxygen concentration.[21] Other strategies that increase risk of further air leak were non synchronized, pressure limited, long inspiratory time ventilation. In preterm with RDS, use of CPAP increased the risk of air leak compared to no respiratory support.[22], [23], [24], [25], [26]
This case report showed spontaneous pneumothorax term newborn with signs of respiratory distress and oxygen desaturation below 50%. Within 1 hour after NICU admission, the target oxygen saturation was maintained with the administration of CPAP. Clinical improvement was observed after 24 hours and oxygen was put off after 48 hours. Repeated chest x-ray on day 4 revealed resolution of pneumothorax, and the neonate was discharged from level-3 NICU on day 5. Previous studies reported approximately 70% to 80% term neonates with pneumothorax resolved without drainage. Arora et al. demonstrated non-invasive management using 100% oxygen via oxygen hood followed by gradual decreases of FiO2 (40-60%) successfully resolved the pneumothorax with no harm reported. [19] In contrast, Lim et al. reported a high rate of chest tube drainage (57%) compared to other studies.[8] The difference in study settings and the proportion of subjects with risk factors might contribute to the high rate of chest tube drainage.
Neonatal pneumothorax remains one of the major causes of neonatal morbidity. In an 8-year regional Danish study, 59% of symptomatic infants required drainage with a significant longer duration of hospitalization, respiratory support, and higher mortality compared to infants without drainage. [10] Infants with both spontaneous pneumothorax and RDS had increased risk of intraventricular hemorrhage, chronic lung disease, and death. [8], [10] In a separate study, persistent pulmonary hypertension was reported among term infants with spontaneous pneumothorax. [17] The mortality rate of spontaneous pneumothorax ranged from 13%-65%, nevertheless, pneumothorax was not the direct cause reported.[7], [10], [17]
In conclusion, spontaneous pneumothorax could occur in term newborn without perinatal risk factors, underlying lung diseases, history of resuscitation, or respiratory support. Therefore, spontaneous pneumothorax should be suspected in any neonates with sudden unexplained deterioration in oxygenation, ventilation, or cardiovascular status. Early recognition is essential, allowing timely diagnosis and preventing undue management and neonatal morbidities.
Source of Funding
None.
Conflict of Interest
None.
References
- NH Hillman, SG Kallapur, AH Jobe. Physiology of transition from intrauterine to extrauterine life. Clin Perinatol 2012. [Google Scholar]
- RM Kliegman, RE Behrman, HB Jenson. . Nelson textbook of pediatrics 2020. [Google Scholar]
- M Hassan, M Begum, S Haque, N Jahan, A Mannan, A Rob. Pneumothorax in Neonate. North Int Med Coll J 2015. [Google Scholar]
- Al Tawil, K Abu-Ekteish, F M Tamimi, Al Hathlol, Al Hathal, A Laimun. Symptomatic Spontaneous Pneumothorax in Term Newborn Infants. Pediatr Pulmonol 2004. [Google Scholar]
- S Katar, C Devecioǧlu, M Kervancioǧlu, R Ülkü. Symptomatic spontaneous pneumothorax in term newborns. Pediatr Surg Int 2006. [Google Scholar]
- B Apiliogullari, GS Sunam, S Ceran, H Koc. Evaluation of neonatal pneumothorax. J Int Med Res 2011. [Google Scholar]
- ÍS Silva, F De-Lima, G Rocha, I Alves, H Guimarães. Pneumothorax in neonates: A level III Neonatal Intensive Care Unit experience. J Pediatr Neonatal Individ Med 2016. [Google Scholar]
- HS Lim, H Kim, JY Jin, YL Shin, JO Park, CH Kim. Characteristics of Pneumothorax in a Neonatal Intensive Care Unit. J Korean Soc Neonatol 2011. [Google Scholar]
- M Okumuş, AU Zubarioğlu. Neonatal Pneumothorax - 10 Years of Experience From a Single Center. J Pediatr Res 2020. [Google Scholar]
- L Vibede, E Vibede, M Bendtsen, L Pedersen, F Ebbesen. Neonatal Pneumothorax: A Descriptive Regional Danish Study. Neonatology 2017. [Google Scholar]
- A Ramadanti, I Hendarman. Faktor Risiko Kebocoran Udara Pulmonal pada Neonatus yang Dirawat di Ruang Perawatan Neonatus Intensif Rumah Sakit Mohammad Hoesin Palembang.. Sari Pediatr 2016. [Google Scholar]
- Y Lai, Y Chia, C Wen, H Hsu, H Chang, W Huang. Association between risk of neonatal pneumothorax and mode of anesthesia for cesarean delivery at term: a nationwide population-based retrospective cohort study. Int J Obstet Anesth 2017. [Google Scholar]
- V Zanardo, E Padovani, C Pittini, N Doglioni, A Ferrante, D Trevisanuto. The Influence of Timing of Elective Cesarean Section on Risk of Neonatal Pneumothorax. J Pediatr 2007. [Google Scholar]
- V James, J Rajan. Bilateral spontaneous pneumothorax in a term newborn. Indian J Case Rep 2020. [Google Scholar]
- SK Kim, WH Kim. Tension pneumothorax in a newborn after Cesarean-section delivery - A case report. Korean J Anesthesiol 2010. [Google Scholar]
- SM Adler, I Wyszogrodski. Pneumothorax as a function of gestational age: Clinical and experimental studies. J Pediatr 1975. [Google Scholar]
- J Smith, RE Schumacher, SM Donn, S Sarkar. Clinical course of symptomatic spontaneous pneumothorax in term and late preterm newborns: Report from a large cohort. Am J Perinatol 2011. [Google Scholar]
- MM Gharibvand. Spontaneous pneumothorax in a term neonate: A case report. Asian J Pharm Clin Res 2018. [Google Scholar]
- K Arora, SS Panda, RR Das, PK Mohanty, M Panda. Primary spontaneous bilateral pneumothorax in a neonate. APSP J Case Rep 2014. [Google Scholar]
- RC Reed, BL Waters, JR Siebert. Complications of percutaneous thoracostomy in neonates and infants. J Perinatol 2016. [Google Scholar]
- TH William, RA Ballard, ME Avery, CA Gleason. . Avery’s diseases of the newborn 2005. [Google Scholar]
- C Kamlin, PG Davis. Long versus short inspiratory times in neonates receiving mechanical ventilation. Cochrane Datab Syst Rev 2003. [Google Scholar]
- JJ Ho, P Subramaniam, PG Davis. Continuous distending pressure for respiratory distress in preterm infants. Cochrane Datab Syst Rev 2015. [Google Scholar]
- TP Stevens, M Blennow, EW Myers, R Soll. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev 2007. [Google Scholar] [Crossref]
- C Klingenberg, W Ki, N Mccallion, D Pg. Volume-targeted versus pressure-limited ventilation in neonates. Cochrane Database Syst Rev 2017. [Google Scholar] [Crossref]
- A Greenough, TE Rossor, A Sundaresan, V Murthy, AD Milner. Synchronized mechanical ventilation for respiratory support in newborn infants. Cochrane Database Syst Rev 2016. [Google Scholar] [Crossref]
