- Visibility 107 Views
- Downloads 20 Downloads
- DOI 10.18231/j.ijpns.2023.019
-
CrossMark
- Citation
Trailblazing exploration: Unraveling phlebitis and its enigmas in children
- Author Details:
-
Ramgopal Sharma *
-
M.S. Cecilia
-
Latha Venkatesan
-
Kanaram Jat
Introduction
Hospitalized children are given various drugs, fluids, blood, nutritional product, and many other things to treat them. Medicines are given through various routes of drug administration e.g. oral, intradermal, subcutaneous, intravenous and intraosseous, etc. The intravenous route is the most common, effective, and essential method of drug administration in children to manage emergency conditions as well as stable conditions.
There are various devices to administer drugs via the intravenous route e.g. Peripheral intravenous cannula (PIVC), Central Intravenous Catheter (CIVC) peripherally inserted central catheter (PICC), subcutaneously implanted devices, etc. Among all these devices PIVC is the most common device which is used to give medicine to sick children. It is used in any small health center as well as tertiary hospitals. It is cheaper and more convenient to establish compared to other devices which are used for the same purpose.
The whole procedure of PIVC insertion takes a few minutes that’s why it is most commonly used in all emergency conditions. Although insertion of PIVC is easier, it also requires enough skill for its placement and maintenance. Especially in children insertion of PIVC is more difficult, because their veins are so fragile and narrow bore.[1] Maintenance of PIVCs in children is challenging also because of various factors such as the inability to do proper dressing or fixation because of the small size of extremities or site of PIVC insertion, less cooperation from child and parents, etc.[2]
PIVCs are having many advantages, like being easy to insert low cost, requiring less time to establish, convenient to handle, make less discomfort to the patient whereas these are having some disadvantages also as phlebitis, infiltration, extravasation, accidental removal, etc.[3], [4] Predisposing factors for PIVC associated phlebitis are puncture failure, previous complications related to the blood vessel, drugs administration and solutions with pH extremes and osmolarity,[5] history of the previous infiltration, PIVC readjustment during insertion, use of infusion pump for giving medicine, intermittent drug administrations, shorter dwell time,[4] joint involvement, indwelling time [1] size of catheter, location and length of infusion[6] use of antibiotics and potassium chloride[7] neonatal age,[8] etc.
Back ground of the study
More than 80% of hospitalized children require intravenous access at some stage during their admission, and worldwide more than one billion PIVCs are used annually.[9] PIVC allows fluids, medications, etc to be introduced directly into the cardiovascular system and reach most target organs very quickly. Once established, a well-functioning PIVC can remain in use for many days.[9]
Phlebitis is the commonest complication of PIVCs. Phlebitis is defined as an inflammation of tunica intima which is the innermost layer of the blood vessel, [8] Common types of phlebitis are- chemical phlebitis, caused due to the administration of various medications and solutions or fluids, mechanical phlebitis occurs because of trauma caused by the catheter in the tunica intima and infectious phlebitis is called when it resulted after infusion of contaminated solution or infection at the catheter insertion site or handled with aseptic techniques.[8] Phlebitis that occurs after the removal of PIVC is called post-infusion phlebitis. It may occur between 48 to 96 hours after PIVC removal.[8] Sign and Symptoms of phlebitis are pain, erythema, edema, induration, local heat, palpable venous cord in the path of the vessel, and purulent exudates at the puncture site.[1], [5]
The pathophysiology of phlebitis is that when a child gets punctured for PIVC insertion, the risk of infection occurs. Piercing the skin allows bacteria and pathogens enter to the bloodstream. Therefore, the risk of bacteremia increases.[10] Common pathogen which is responsible for infectious phlebitis is coagulase-negative staphylococcus which is found over children’s skin as a normal flora.[8]
Neonates and infants compared to older children have five times more chances of developing phlebitis. Children who are administered IV medication have double the risk of developing PIVC-associated phlebitis. Longer duration of PIVC in situ is also considered a risk factor for developing phlebitis.[8]
Need of the study
Many studies have been done on adult patients related to phlebitis but limited studies are available that were done on children. Studies that were done on children showed the incidence of PIVC associated phlebitis from 2.7 % [5] to 71 %.[3] There is a huge gap between these findings. One study showed that age, size of cannula, site of the cannula, joint involvement, and duration of the cannula in situ had no significant association with PIVC-associated phlebitis where as another study had considered all these as risk factors for phlebitis. The findings of these studies are contradictory so more studies are required to discover the fact.
Materials and Methods
It was a quantitative, prospective and observational study which was conducted in two pediatrics wards at the All India Institute of Medical Sciences (AIIMS) in New Delhi. Hospitalized children with PIVC were enrolled in the study who met inclusion criteria. Sampling technique was purposive sampling. This study included children who were having only one PIVC at a time, up to 12 years of age, willing to participate in the study. This study excluded children who were on continuous sedation, on continuous analgesics infusion, previously enrolled in the study, PIVC inserted more than 12 hours before enrolment.
Sample size
We calculated sample size by using Cochran’s formula. Where we took level of precision 5% and Confidence level 90% because at 95% confidence interval sample size was coming very high which was not possible to achieve in avail period of data collection. Prevalence was considered 71% which was derived from previous study. Sample size was came 222.86 which was rounded of to 225.
Ethical Consideration
Ethical approval was obtained from the institute ethics committee, AIIMS, New Delhi. Informed consent from parents and assent from children were taken before the study. The confidentiality and anonymity of the participants were maintained throughout the study period. Information collected from participants was used only for research purposes. This study was registered in CTRI and registration number was CTRI/2022/06/043603.
Tool for data collection
We used three tools to collect the data. Tool one was used to collect demographic details and clinical profile of participants which was developed by the researcher. This tool consists of eight items related to demography that includes name, age, gender, height, weight, education of child's mother, education of child's father, and 2 items related to clinical profile including the history of previous hospitalization and primary diagnosis. Content validity of tool-1 was obtained from seven experts. There was 100 % agreement among the expert. Tool-2 was a semi-structured tool to assess predisposing factors which was also developed by the researcher to assess predisposing factors of phlebitis. It consists of 15 items including Extremity and Site of PIVC, Size of PIVC, Fixation of PIVC, Use of Splint and three ways Extensions, PIVC inserted by, Number of injection per day, Method of drugs administration, name of antibiotics, name of other drugs crystalloid, colloid, Electrolytes correction fluid and cancer drugs, Frequency of PIVC handling. Content validity of tool was obtained from seven experts. There was 100 % agreement among the experts. Tool-3 was Jackson's visual infusion phlebitis (VIP) scale which was a standard tool to identify phlebitis. Permission was taken from the developer of the tool.
Pilot study
The pilot study was conducted on twenty children for assessing the feasibility of the study. Children who were enrolled in pilot study were excluded from final study.
Data collection procedure
Total 679 children were screened, out of which 454 were excluded on the basis of various exclusion criteria and 225 were enrolled for study. Data was collected from 15 July 2022 to 30 November 2022. Demographic data was collected by interviewing the care giver. Information on predisposing factors was obtained from medical records and by observation. The PIVC site was assessed at the same time each day till phlebitis developed or maximum for seven consecutive days.The findings of the assessment were recorded as per the VIP Scale criteria of phlebitis.
Data analysis
Data were analyzed using SPSS 26.0 version. The demographic and clinical variables were described using frequency, percentage, mean, standard deviation, median, and range. As part of inferential statistics, the Chi-square test was used for inference at a 0.05 level of significance.
Result
Majority of children (50.7%) were from the 6-12 year age group, the mean age was 6.16±1.09 years, and children were aged from 25 days to 12 years. The majority of children (64.44%) were male. Mean height of children was 104.47±25.30 cm and the range was 44 - 159 cm. The mean weight was 17.52±8.87 kg and the range was 1.94-56 kg. The majority of children (63.66%) had a history of previous hospitalization. The majority of children (25.77%) were diagnosed with malignancies. Characteristics of children given in [Table 1].
|
S. No. |
Socio-demographic characteristics |
Frequency (%) |
|
1. |
Age (years) |
|
|
|
0- 1 |
21 (9.3) |
|
|
1-3 |
36 (16) |
|
|
3-6 |
54 (24) |
|
|
6-12 |
114 (50.7) |
|
Mean ±SD |
6.16±1.09 |
|
|
|
Range |
25 days- 12 year |
|
2. |
Gender |
|
|
|
Male |
145 (64.44) |
|
|
Female |
80 (35.56) |
|
3 |
Height |
104.47±25.30*, 44 -159# cm |
|
4 |
Weight |
17.52±8.87*, 1.94 -56# kg |
|
5 |
History of the Previous hospitalization |
|
|
|
No |
82(36.34) |
|
|
Yes |
143 (63.66) |
|
6 |
Primary Diagnosis |
|
|
|
Malignancies |
58 (25.77) |
|
|
Renal Disorders |
56 (24.88) |
|
|
Gastroenterological Disorders |
31 (13.75) |
|
|
Neurological Disorders |
24 (10.90) |
|
|
Respiratory Disorders |
38 (16.7) |
|
|
Autoimmune Disorders |
6 (3.7) |
|
|
Others Disorders |
11(4.3) |
Majority of PIVC (45%) were inserted in the left upper extremity. The majority of PIVCs (43.2%) were inserted in the dorsum of the hand. Joint was involved with most of the PIVCs (79.55%). Most of the PIVCs (63.11%) were of 24 gauge size. More than half (59.11%) of PIVC were fixed with the use of transparent and non-transparent adhesives together. The splint was not used in the majority of PIVCs (89.44%) Three-way was not used in most of the PIVCs (74.33%). Most of the PIVCs (77.33%) were inserted by junior resident doctors. Dressing of most of the PIVCs (91.55%) was clean. Accessories (IV set, Syringe, and Pressure line) were changed every day in all of the children. In the majority of children (37.77%) PIVC was handled thrice a day. In more than half (54.22%) of children reason for PIVC removal was phlebitis. In more than half of children (57.8%), medicines were given by using more than one method of drug administration e.g. bolus, syringe pump, and IV drip set. More than half of the children (56.9%) received a maximum of two injections per day. Crystalloid fluids were not given to most of the children (47.55%). Majority of children (85.77%) did not receive colloid fluids.
|
S No. |
PIVC and medicine related characteristics |
Frequency (%) |
|
1 |
Extremity in which PIVC has inserted |
|
|
|
Right Upper Extremity |
91 (40.5) |
|
|
Left Upper Extremity |
103 (45) |
|
|
Right Lower Extremity |
10 (4.8) |
|
|
Left Lower Extremity |
21 (9.7) |
|
2 |
Site of PIVC |
|
|
|
Dorsum of Hand |
99 (43.2) |
|
|
Forearm |
66 (29.3) |
|
|
Antecubital Fossa |
28 (12.2) |
|
|
Dorsum of Foot |
23 (11.4) |
|
|
Leg |
9 (3.9) |
|
3 |
Joint involvement with the PIVC site |
|
|
|
Yes |
179 (79.55) |
|
|
No |
46 (20.45) |
|
4 |
Size of PIVC |
|
|
|
26 Gauge |
31 (13.8) |
|
|
24 Gauge |
142 (63.11) |
|
|
22 Gauge |
43 (19.10) |
|
|
20 Gauge |
8 (3.59) |
|
|
18 Gauge |
1(0.4) |
|
5 |
Fixation of PIVC done |
|
|
|
Transparent Adhesive |
91 (40.45) |
|
|
Non Transparent Adhesive |
1 (0.44) |
|
|
With Both |
133(59.11) |
|
6 |
Splint used in PIVC Fixation. |
|
|
|
Yes |
24 (10.56) |
|
|
No |
201 (89.44) |
|
7 |
Three ways Extensions (10 cm length) connected with PIVC |
|
|
|
Yes |
58(25.67) |
|
|
No |
167 (74.33) |
|
8 |
PIVC inserted by |
|
|
|
Nursing Officer |
28 (12.44) |
|
|
JR |
174 (77.33) |
|
|
SR |
23 (10.23) |
|
9 |
Dressing of PIVC |
|
|
|
Clean |
206 (91.55) |
|
|
Dirty |
19 (8.45) |
|
10 |
Accessories changed /day Yes |
225 (100) |
|
11 |
Frequency of PIVC handling |
|
|
|
Once in A Day |
65 (28.89) |
|
|
Twice In A Day |
30 (13.33) |
|
|
Thrice in A day Four Times in day |
85 (37.78) 45 (20.0) |
|
12 |
Reason for PIVC removal |
|
|
|
Phlebitis |
122 (54.22) |
|
|
Discharged |
78 (34.7) |
|
|
Blocked |
8 (3.6) |
|
|
Leaking |
5 (2.2) |
|
|
Accidental Removal |
4 (1.8) |
|
|
Two PIVCs were inserted after enrollment |
8 (3.48) |
|
13 |
Administration of medicine |
|
|
|
By Bolus |
19 (8.4) |
|
|
By using a syringe pump |
45 (20.0) |
|
|
By using the IV Drip set |
31 (13.8) |
|
|
By more than one |
130 (57.8) |
|
14 |
No. of injections per day |
|
|
|
No injection |
20 (8.9) |
|
|
Less than 2 |
128 (56.9) |
|
|
3-4 |
51 (22.7) |
|
|
5-6 |
15 (6.7) |
|
|
> 6 |
11 (4.9) |
|
15 |
Administration of Crystalloid fluid |
|
|
|
No crystalloid |
107 (47.55) |
|
|
N/2 with5% Dx |
30 (13.3) |
|
|
N/5 with5% Dx |
4 (1.8) |
|
|
DNS |
80 (35.55) |
|
|
NS |
4 (1.8) |
|
16 |
Administration of Colloid fluid |
|
|
|
No Colloid |
193 (85.77) |
|
|
Albumin |
26 (11.53) |
|
|
IVIG |
4 (1.8) |
|
|
Blood |
2 (0.9) |
|
17 |
Electrolytes correction |
|
|
|
No Correction |
203 (90.22) |
|
|
Kcl |
3 (1.33) |
|
|
NaHCo3 |
10 (4.45) |
|
|
Calcium |
9 (4) |
|
18 |
Antibiotics |
|
|
|
No |
80 (35.45) |
|
|
Yes |
145 (64.55) |
|
19 |
Other drugs |
|
|
|
No |
85 (37.78) |
|
|
Yes |
140 (62.22) |
|
20 |
Cancer Drug |
|
|
|
No |
199 (88.44) |
|
|
Yes |
26 (11.56) |
|
S. No |
Variable |
Phlebitis 122 (54.4) |
No Phlebitis 103(45.6) |
X2 Value |
P Value |
|
1 |
Gender |
|
|
.537 |
.277 |
|
|
Male |
76 (62.3) |
69 (67) |
|
|
|
|
Female |
46 (37.7) |
34(33) |
|
|
|
2 |
Age |
|
|
4.224 |
.065 |
|
|
0- 1 year |
11 (9.01) |
10 (9.70) |
|
|
|
|
1-3 year |
14 (11.47) |
22 (21.35) |
|
|
|
|
3-6 year |
26 (21.31) |
28 (27.18) |
|
|
|
|
6-12 year |
71 (58.19) |
43 (41.74) |
|
|
|
3 |
Previous Hospitalization |
|
|
9.636 |
.239 |
|
|
No |
42(34.4) |
40(38.80) |
|
|
|
|
Yes |
80(65.57) |
63(61.16) |
|
|
|
4 |
Primary Diagnosis |
|
|
9.948 |
.169 |
|
|
Malignancy |
33(27) |
24(23.3) |
|
|
|
|
Renal Disorder |
24(19.7) |
33(32) |
|
|
|
|
Gastroenterological disorder |
19(15.6) |
12(11.7) |
|
|
|
|
Neurological Disorder |
16(13.1) |
8(7.8) |
|
|
|
|
Reparatory Disorders |
22(18.8) |
16(15.5) |
|
|
|
|
Autoimmune Disorders |
1(0.8) 6(4.9) |
5(4.9) |
|
|
|
|
Others Disorders |
|
5(4.9) |
|
|
|
5 |
Extremity of PIVC |
|
|
3.673 |
.291 |
|
|
Right Upper |
49(40.2) |
42(40.8) |
|
|
|
|
Left Upper |
56(45.9) |
47(45.6) |
|
|
|
|
Right Lower |
8(6.6) |
2(1.9) |
|
|
|
|
Left Lower |
9(7.4) |
12(11.7) |
|
|
|
6 |
Site of PIVC |
|
|
|
|
|
|
Dorsum of Hand |
50(41) |
49(47.6) |
|
|
|
|
Forearm |
38(30.4) |
28(27.2) |
|
|
|
|
Antecubital Fossa Dorsum of Foot |
18(14.8) 9(7.4) |
10(9.7) 14(13.6) |
|
|
|
|
Leg |
7(5.7) |
2(1.9) |
6.227 |
.266 |
|
7 |
Joint Involvement |
|
|
2.816 |
.100 |
|
|
Yes |
92(75.4) |
87(84.5) |
|
|
|
|
No |
30(24.6) |
16(15.5) |
|
|
|
8 |
Size of PIVC |
|
|
5.963 |
.169 |
|
|
Gauge 26 |
12(9.8) |
19(18.4) |
|
|
|
|
Gauge.24 |
77(63.1) |
65(63.1) |
|
|
|
|
Gauge 22 |
28(23) |
15(14.6) |
|
|
|
|
Gauge.20 |
4(3.3) |
4(3.9) |
|
|
|
|
Gauge 18 |
1(0.8) |
0(0) |
|
|
|
9 |
Fixation of PIVC |
|
|
1.041 |
.828 |
|
|
Transparent Adhesive |
51(41.8) |
40(38.8) |
|
|
|
|
Non Transparent Adhesive |
1(0.8) |
0(0) |
|
|
|
|
With Both |
70(57.4) |
63(61.2) |
|
|
|
10 |
Use of Splint |
|
|
1.0 |
.581 |
|
|
Yes |
12(10.7) |
11(10.7) |
|
|
|
|
No |
109(89.3) |
92(89.3) |
|
|
|
11 |
Use of Three-way |
|
|
5.705 |
.037 |
|
|
Yes |
38(31.1) |
19(18.4) |
|
|
|
|
No |
84(68.9) |
83(80.6) |
|
|
|
12 |
Condition of PIVC Dressing |
|
|
2.423 |
.485 |
|
|
Dry and Clean |
109(89.3) |
97(94.2) |
|
|
|
|
Dry and Dirty |
13(10.65) |
6(5.8) |
|
|
|
13 |
Person who put PIVC |
|
|
3.494 |
.481 |
|
|
Nursing Officer |
19(15.6) |
9(8.7) |
|
|
|
|
JR |
89(72.95) |
85(82.52) |
|
|
|
|
SR |
14(11.5) |
9(8.7) |
|
|
No electrolyte correction fluid was given to most of the children (90.22%). Majority of the children (64.55%) received antibiotics. Many other drugs (analgesics, PPI, anticonvulsants, antiemetics, steroids, etc) are also used in the majority of children (62.22%). Cancer drugs were not received by majority of the children (88.44%).([Table 2]).
In this study we found the incidence of phlebitis 54.22% at 95% confidence interval. 122 out of 225 children devolved PIVC-associated phlebitis. There was no statistically significant association between age, gender, history of previous hospitalization, primary diagnosis, extremity of PIVC, site of PIVC, joint involvement, size of PIVC, fixation of PIVC, and use of splint with the incidence of phlebitis. Use of three way found statistically significant association with phlebitis. Condition of PIVC dressing and person who inserted PIVC found no significant association with phlebitis. ([Table 3])
There was a statistically significant association between method of drug administration and phlebitis. No association was found between the number of injections and phlebitis. A statistically significant association was found between crystalloid fluids and phlebitis. Electrolytes correction fluids, colloid fluids, and antibiotics found no association with phlebitis. Frequency of PIVC and cancer drugs was found statistically significant association with phlebitis.([Table 4]).There was no statistically significant association between the duration of PIVC in situ and phlebitis.([Table 5]).
|
S. No |
Variable |
Phlebitis 122 (54.4) |
No Phlebitis 103(45.6) |
X2 Value |
P Value |
|
1 |
Method of drug administration |
|
|
8.807 |
.031 |
|
|
By Bolus |
5(4.1) |
14(13.6) |
|
|
|
|
By using a syringe pump |
22(18) |
23(22.3) |
|
|
|
|
By using IV Drip set |
16(13.1) |
15(14.6) |
|
|
|
|
By more than one |
79(64.8) |
51(49.5) |
|
|
|
2 |
Number of Injection |
|
|
8.854 |
.062 |
|
|
No injection |
7(5.7) |
13(12.6) |
|
|
|
|
Less than 2 |
66(54.1) |
62(60.2) |
|
|
|
|
3 -4 |
29(23.8) |
22(21.4) |
|
|
|
|
5-6 |
12(9.8) |
3(2.9) |
|
|
|
|
>6 |
8(6.6) |
3(2.9) |
|
|
|
3 |
Crystalloid fluid |
|
|
12.647 |
.016 |
|
|
Yes |
74(60.65) |
44(42.70) |
|
|
|
|
No |
48(39.35) |
59(57.30) |
|
|
|
4 |
Electrolyte correction fluid |
|
|
2.763 |
0.96 |
|
|
Yes |
15(12.3) |
6(5.8) |
|
|
|
|
No |
107(87.7) |
97(94.2) |
|
|
|
5 |
Colloid Fluid |
|
|
.671 |
.371 |
|
|
Yes |
16(13.11) |
87(84.5) |
|
|
|
|
No |
106(86.9) |
16(15.53) |
|
|
|
6 |
Antibiotics |
|
|
.162 |
.086 |
|
|
Yes |
84 (68.9) |
42(40.8) |
|
|
|
|
No |
38 (31.1) |
61(59.2) |
|
|
|
7 |
Frequency of PIVC handling |
|
|
14.364 |
.004 |
|
|
OD |
25(20.5) |
39(37.9) |
|
|
|
|
BD |
15(12.3) |
15(14.6) |
|
|
|
|
TDS |
49(40.2) |
36(35) |
|
|
|
|
QID |
33(27) |
12(11.7) |
|
|
|
8 |
Cancer Drugs |
|
|
14.593 |
.001 |
|
|
NO |
103(84.4) |
96(93.2) |
|
|
|
|
Yes |
19(15.6) |
7(6.8) |
|
|
|
S. No |
Variable |
Phlebitis 122 (54.4) |
No Phlebitis 103(45.6) |
X2 Value |
P Value |
|
1 |
Duration of PIVC in situ |
|
|
7.405 |
.060 |
|
Less than 24 Hours |
6 (4.9) |
2 (1.9) |
|||
|
24 - 48 Hours |
33 (27.0) |
15 (14.7) |
|||
|
48 -72 Hours |
44 (36.1) |
43 (41.7) |
|||
|
More than 72Hours |
39 (32.0) |
43 (41.7) |
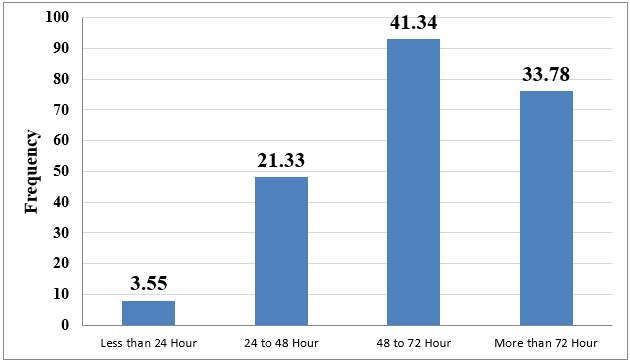
Children who got medicine via a syringe pump are having 3.6 times more chance to develop phlebitis and it is statistically significant. Crystalloid is a risk factor for phlebitis but it is not statistically significant. Children, who got medicine OD, have less chance to develop phlebitis. It is statistically significant. Children who got cancer drugs have a 3.47 times more chance to develop phlebitis. It is statistically significant. Children, in whom three-way was not used, have less chance to develop phlebitis but it was not statistically significant.([Table 6] ). The Mean±SD life span was 69.86 ±32.88 hours, the minimum life span was 19 hours and the maximum was 180 hours. We categorized whole life span in four categories. The majority of PIVCs (41.34%) remained in situ for 48-72 hours only 3.55 % of PIVCs were removed within 24 hours.([Figure 1]).
|
S. No |
Variables |
AOR |
P-value |
95 % Confidence Interval |
|
|
1 |
Method of drug administration |
1 |
|
|
|
|
|
Bolus |
3.633087 |
0.045 |
1.031514 |
12.79607 |
|
|
Syringe pump |
|
0.357 |
.4813347 |
7.585859 |
|
|
IV set |
1.910847 |
0.205 |
.6567154 |
7.077204 |
|
|
More than one method |
2.155855 |
|
|
|
|
2 |
Crystalloid Fluids |
|
|
|
|
|
|
No |
1 |
|
|
|
|
|
Yes |
1.867679 |
.080 |
.9284017 |
3.757235 |
|
3 |
Frequency of PIVC handling |
|
|
|
|
|
|
OD |
.1586308 |
.000 |
.0613259 |
.4103279 |
|
|
BD |
|
.134 |
.161316 |
1.275465 |
|
|
TDS |
.4535999 |
.088 |
.2136735 |
1.113918 |
|
|
QID |
.48786751 |
|
|
|
|
4 |
Use of cancer drug |
|
|
|
|
|
|
No |
1 |
|
|
|
|
|
Yes |
3.478134 |
.018 |
1.235366 |
9.792583 |
|
5 |
Use of three-way extension |
|
|
|
|
|
|
Yes |
1 |
|
|
|
|
|
No |
.8706736 |
0.771 |
.4188998 |
1.809675 |
Discussion
In the present study incidence of phlebitis was 54.22% which is contrary to the findings of Urbanetto et al (2016) [11] reported 1.25%, Jacinto et al(2014) [5] reported 2.7%, Foster et al (2002) [8] reported 6.6%, Salma U et al (2019) [7] reported18.09 % whereas these findings are similar as reported by Sijabat et al (2021) [12] 64 % and Nagpal et al (2015) [13] 71.25%. This gap between the findings might be due to sampling size, population, setting, methodology, and PIVC care. According to Infusion Therapy Standards of Practice, 5% [14] incidence of phlebitis is acceptable but in the present study, the incidence of phlebitis was more than 10 times.
In this present study, 62.3 % of phlebitis has occurred in males and 37.7 % in females but no significant association was found between gender and phlebitis. The findings of the present study were in accordance with the study finding of Jacinto et al (2014) [5] which showed no association between gender and phlebitis, these findings are also in line with the findings of Tefera et al (2020)[1] which showed no association between gender and phlebitis but the findings of the present study were in contrast with the study findings of Sijabat M et al. (2021) [12] which showed that male gender is a risk factor for phlebitis and the study done by Luyu et al(2020) [15] in which author found an association between gender and phlebitis.
The present study found that the majority of phlebitis (58.19%) occurred in 6-12 years of age children and the minimum (9.01%) in 0-1 year of age. No significant association was found between age and phlebitis. This finding is in contrast with the findings reported by Foster L et al.(2002) [8] in their study that the smaller age of children is a risk factor for developing phlebitis.
Children with a history of hospitalization may have some knowledge regarding PIVC and its care which may have an impact on phlebitis. In the present study, the majority of phlebitis (65.57%) occurred in children who had a history of previous hospitalization but it was not statistically significant. No other study reported about association between phlebitis and a history of previous hospitalization.
The present study findings suggest that more than half of children with an incidence of phlebitis were diagnosed with various malignancies and renal disorders. In this present study, no association was found between primary diagnosis and phlebitis and this finding is in contrast with the study finding of Abdelaziz RB et al (2017), [16] who suggested that respiratory disorder and infections are the risk factors for phlebitis.
The present study shows that there is no association between extremities, site of PIVC, and phlebitis. These findings are similar to those published by Tefera et al (2020) [1] in their study but these findings are also in contrast with the findings published in a meta-analysis done by Luyu et al (2020) [15] in which the forearm was considered as a risk factor for phlebitis.
The present study demonstrates that 75.4 % incidence of phlebitis occurred in those children in whom the joint was involved at the PIVC site. In the present study, no significant association was found between phlebitis and joint involvement at the PIVC site. These findings are in contrast with the study findings conducted by Tefera et al (2020) [1] which described that involvement of joint at PIVC site is a risk factor for phlebitis.
The present study depicts that there is no significant association between phlebitis and the size of PIVC. These findings are contrary to the findings of Abdelaziz et al (2017), [16] which showed that 24 size cannula is a risk factor for phlebitis.
The present study found that more than half (57.4) PIVCs were fixed with transparent adhesive, then non-transparent adhesive was also applied over the transparent adhesive to resecure that. The fixation of PIVC found no association with phlebitis and similar findings were reported by Salma et al (2019) [7] in their study.
Most of the incidence of phlebitis (89.3%) occurred in those children in whom a splint was not used to stabilize the PIVC. The use of a splint found no association with phlebitis and a similar finding was published in a study, done by Abdelaziz et al (2017). [16]
This study found that majority of PIVC dressing (89.3%) was dry and clean. No significant association was found between the condition of PIVC dressing and phlebitis while Abdelaziz et al (2017) [16] found that dressing plays a vital role in PIVC complications.
Most of the PIVC (72.95%) was inserted by a junior resident doctor. No association was found between phlebitis and the person who inserted phlebitis. No study reported the association between phlebitis and the person who inserted the PIVC.
The present study found that there is a significant association between the use of three-way extension and phlebitis. Regression analysis showed that the use of three-way is a protective factor for phlebitis. No study has reported any findings about three-way use.
In the majority of children (64.8%) drugs were given by using multiple methods of drug administration at a time including bolus, syringe pump, and IV drip set. Method of drug administration found a significant association with phlebitis. These findings are in line with findings reported by Abdelaziz et al (2017) [16] in their study that the use of a syringe pump is a risk factor. But the findings of the present study are also in contrast with a study done by Jacinto et al (2014) [5] found no association between the method of drug administration and phlebitis.
The finding of the present study depicts that more than half of the children (56.88%) were administered less than two injections per day. No significant association was found between the number of injections per day and phlebitis. There is no study which reported similar findings.
The present study revealed that 60.65% of children who developed phlebitis got crystalloid fluids. The association between phlebitis and the use of crystalloids was significant. These findings are consistent with the study conducted by Jacinto et al(2014) [5] in which fluids are a risk factor. The findings are also consistent with the study done by Parul et al (2015) [3] on the clinical pattern of phlebitis among children showed crystalloids increase the risk of phlebitis.
In the present study, 14.23 % of children received colloids. Three colloids were given to patients including IVIG, Albumin, and Blood. The present study found no association between phlebitis and colloid. These findings are similar to the findings of the study conducted by Mandal et al (2019) [17] where they reported that no significant association between blood transfusion and phlebitis. No study has reported the association between IVIG or Albumin with phlebitis.
The present study depicts there is no association between phlebitis and electrolyte correction fluid including kcl, calcium gluconate, and sodium bicarbonate. In the present study analysis of each electrolyte was impossible as more than one electrolyte was administered to the children. These findings are in contrast with the study done by Bitencourt et al (2018) [2] which showed an association between kcl and phlebitis. No study has reported any findings about the association between calcium gluconate or sodium bicarbonate, and phlebitis.
This present study found no association between the use of antibiotics and phlebitis. These findings are contrary to the study conducted by Bitencourt et al (2018) [2] which found a significant association between antibiotics and phlebitis.
The present study reveals that there is a significant association between number of PIVC handling and phlebitis. Regression analysis showed that once in a day PIVC handling is a protective factor for phlebitis. No study has reported the association between the frequency of PIVC handling and phlebitis.
The present study describes that the use of cancer drugs and phlebitis has a significant association. Cancer drugs are also a risk factor for phlebitis. Similar findings were not reported in other studies.
This present study revealed that the mean life span of PIVC was 69.86 ±32.88 hours (range 19- 180 hours). These findings are similar to the findings of Abdelaziz et al (2017) [16] showed a lifespan of 68.82±35.71 hours ( range1-168).
This study reveals that out of the 54.22 % (122) incidence of phlebitis, 36.1% occurred between 48-72 and 32% after 72 hours of placement of PIVC. No significant association was found between the duration of PIVC in situ and phlebitis. These findings are in contrast with the findings of the meta-analysis done by Luyu et al (2020) [15] in which the long duration of PIVC was considered as a risk factor and also with the study conducted by Tefera et al (2020) [1] which described a significant association between phlebitis and 48-72 hour and 72-96 hour of PIVC in situ.
Conclusion
The incidence of phlebitis is much higher than the acceptable limit as per Infusion Therapy Standards of Practice which is to be reduced. Crystalloids, the Method of drug administration, and cancer drugs are the risk factors for phlebitis. The frequency of PIVC handling and use of three-way extension is a protective factor for phlebitis the study suggests that most likely phlebitis occurs after three days.
Strength of study
We have studied 24 possible predisposing factors of phlebitis in this present study. This large number of factors has not been assessed in a single study so far. It is an observational study in which data was collected by a single person hence the risk of bias is the least. Standardized tool was used.
Limitations
Limitations of this study were that this study was limited to only two pediatric wards. Multiple antibiotics were given at a time so individual analysis of antibiotics was not possible. There were more than 80 disease conditions that were diagnosed in enrolled children so individual analysis was not possible. More than 40 drugs were given apart from colloids, crystalloids, and antibiotics so individual analysis could not possible.
Recommendation
We recommend that a similar study can be done with a large sample size. A comparative study also can be done between adult and pediatric populations to find out the incidence of phlebitis. A study can be done to assess the incidence of phlebitis with different classes of drugs like antibiotics, PPI, anticonvulsants, electrolyte connection fluids, colloids, etc.
Source of Funding
None.
Conflict of Interest
None.
References
- M Tefera, S Letta, A Ararsa, M Leta, A Abrham. . Incidence and Its Associated Factors of Phlebitis among Pediatric Patients with Peripheral Intravenous Cannula at Hiwot Fana Specialized University Hospital . [Google Scholar]
- ES Bitencourt, CN Leal, R Boostel, Vda Mazza, JVC Felix, PE Prevalência. Prevalência De Flebite Relacionada Ao Uso De Dispositivos Intravenosos Periféricos Em Crianças. Open 2018. [Google Scholar]
- P Nagpal, GK Khera, Y Kumar. A study Assess the Clinical Pattern of Phlebitis among children admitted in selected hospital of Ambala. Haryana 2015. [Google Scholar]
- AK De Lima Jacinto, AFM Avelar, M Pedreira. Predisposing Factors for Infiltration in Children Submitted to Peripheral Venous Catheterization. J Infus Nurs 2011. [Google Scholar]
- L Jacinto Ak De, AFM Avelar, A Wilson, PM Da. Phlebitis associated with peripheral intravenous catheters in children: study of predisposing factors.. Esc Anna Nery Rev Enferm 2014. [Google Scholar] [Crossref]
- P Gallant, AA Schultz. Evaluation of a Visual Infusion Phlebitis Scale for Determining Appropriate Discontinuation of Peripheral Intravenous Catheters. J Infus Nurs 2006. [Google Scholar]
- U Salma, M Sarker, N Zafrin, KS Ahamed. Frequency of Peripheral Intravenous Catheter Related Phlebitis and Related Risk Factors: A Prospective Study. J Med 2019. [Google Scholar]
- L Foster, M Wallis, B Paterson, H James. A Descriptive Study of Peripheral Intravenous Catheters in Patients Admitted to a Pediatric Unit in One Australian Hospital. J Infus Nurs 2002. [Google Scholar]
- GB Beecham, G Tackling. Peripheral Line Placement. 2023. [Google Scholar]
- Drp Ventura, Jas Freitas, Jff Lindo. Reliability study of Visual Infusion Phlebitis Score Portuguese European version. Internet]. In Review 2021. [Google Scholar]
- S Urbanetto J De, CG Peixoto, TA May. Incidence of phlebitis associated with the use of peripheral IV catheter and following catheter removal. Rev Lat Am Enfermagem 2016. [Google Scholar]
- M Sijabat, SD Nduru, BA Monaretha, YF Sitanggang, EO Hutasoit. Incidence of Phlebitis Following the Use of Peripheral IV Line at X Hospital. Indones Contemp. Nurs J ICON J 2021. [Google Scholar]
- P Gupta, R Rai, S Basu, M Faridi. Life span of peripheral intravenous cannula in a neonatal intensive care unit of a developing country. J Pediatr Nurs 2003. [Google Scholar]
- . Infusion Therapy Standards of Practice-INS. 2016. [Google Scholar]
- L Lv, J Zhang. The incidence and risk of infusion phlebitis with peripheral intravenous catheters: A meta-analysis. J Vasc Access 2020. [Google Scholar]
- R Ben Abdelaziz, H Hafsi, H Hajji, H Boudabous, B Chehida, A Mrabet. Full title: peripheral venous catheter complications in children: predisposing factors in a multicenter prospective cohort study. BMC Pediatr 2017. [Google Scholar]
- A Mandal, K Raghu. Study on incidence of phlebitis following the use of pherpheral intravenous catheter. J Fam Med Prim Care 2019. [Google Scholar]
How to Cite This Article
Vancouver
Sharma R, Cecilia M, Venkatesan L, Jat K. Trailblazing exploration: Unraveling phlebitis and its enigmas in children [Internet]. J Paediatr Nurs Sci. 2023 [cited 2025 Sep 13];6(3):106-116. Available from: https://doi.org/10.18231/j.ijpns.2023.019
APA
Sharma, R., Cecilia, M., Venkatesan, L., Jat, K. (2023). Trailblazing exploration: Unraveling phlebitis and its enigmas in children. J Paediatr Nurs Sci, 6(3), 106-116. https://doi.org/10.18231/j.ijpns.2023.019
MLA
Sharma, Ramgopal, Cecilia, M.S., Venkatesan, Latha, Jat, Kanaram. "Trailblazing exploration: Unraveling phlebitis and its enigmas in children." J Paediatr Nurs Sci, vol. 6, no. 3, 2023, pp. 106-116. https://doi.org/10.18231/j.ijpns.2023.019
Chicago
Sharma, R., Cecilia, M., Venkatesan, L., Jat, K.. "Trailblazing exploration: Unraveling phlebitis and its enigmas in children." J Paediatr Nurs Sci 6, no. 3 (2023): 106-116. https://doi.org/10.18231/j.ijpns.2023.019
